Meta description (140–160): NANOTEC provides non-animal, ISO-aligned cytotoxicity testing for dietary supplements and nutraceutical ingredients. Get IC50 and cell-viability data via MTT assay, expert guidance, and decision-ready reports for registration and marketing.
SEO Keywords (one line): nanotechnology, nanotec, dietary supplement, safety testing service, dietary supplement testing cytotoxicity testing, nutraceutical safety evaluation, in vitro MTT assay, ISO 10993-5, IC50 determination, non-animal testing Thailand, cell viability test, herbal extract safety, raw material screening, supplement registration data
Service Overview
Dietary supplements and nutraceuticals compete on trust. Whether you formulate with botanical extracts, vitamins, minerals, probiotics, or novel bioactives, you must show that your product is safe at the intended use level. Robust in vitro methods deliver that evidence quickly and ethically without animal testing.
At the National Nanotechnology Center (NANOTEC), our Cytotoxicity Testing for Dietary Supplements gives manufacturers, brand owners, and ODM/OEM partners quantitative, defensible safety data built on internationally recognized frameworks. Results from this service % cell viability and IC50 (the concentration that reduces viability to 50%) help you screen ingredients, set safe formulation ranges, compare suppliers, and prepare documentation for internal QA, product registration, and partner dossiers.
This offering forms Safety module within our broader testing support for supplements, which also covers absorption and bioactivity evaluations. Here we focus on cytotoxicity, the essential first step for de-risking your formulation.
Who This Service Is For
- Supplement & nutraceutical brands that need non-animal safety evidence for new launches, reformulations, and tender submissions.
- Ingredient suppliers seeking third-party screening to differentiate botanical extracts or standardized actives.
- ODM/OEM manufacturers who must pre-qualify raw materials and provide customers with concentration guidance.
- Startups and SMEs that want cost-effective, ISO-aligned data to support early market entry.
- Research groups transitioning lab discoveries into commercializable health products.
Why Choose NANOTEC
1) Expert consultation, not just a test.
Our researchers plan the study with you, sample handling, dilution/extraction, and dose design so the data reflect real-world product use (e.g., capsule content diluted in aqueous media; oil-soluble actives solubilized appropriately).
2) Standards-based, non-animal methods.
We design assays with reference to ISO 10993-5 (in vitro cytotoxicity) and use the MTT colorimetric readout, a widely accepted measure of cell viability.
3) Clear, decision-ready reporting.
Data tables, dose-response plots, and an interpretive summary let QA teams, regulatory reviewers, and commercial partners quickly understand what the results mean for your formula.
4) End-to-end support.
From scoping and sample preparation through to result interpretation and optional next-step testing (e.g., absorption or bioactivity modules), our team streamlines your evidence-building journey.
Scope & Pricing
Safety: Cytotoxicity Test (from THB 24,000 per item)
Final pricing depends on the number of samples, concentration range, replication, and any special handling (e.g., complex matrices, highly colored materials). You’ll receive a formal quotation after scoping.
Suitable sample types
- Raw actives: vitamins, minerals, amino acids, polyphenols, carotenoids, etc.
- Botanicals & herbal extracts: solvent extracts, CO₂ extracts, standardized fractions.
- Finished products: powders, capsules/tablets (contents), liquids, syrups, emulsions.
- Functional blends designed for capsules, sachets, shots, and gummies.
The Cytotoxicity Test in Detail
Purpose
To determine whether a test article adversely affects the viability of cultured cells under defined exposure conditions, and where possible, to estimate IC50 to guide safe use levels in formulations.
Principle (MTT Assay)
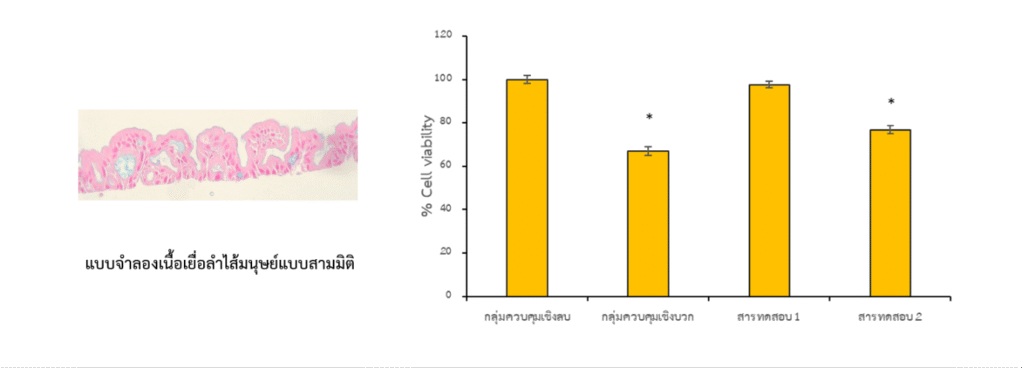
Cells are grown in culture and exposed to a dose-response series of the test article. Viable cells convert MTT into a purple formazan product. Absorbance is measured, normalized to controls, and used to calculate % cell viability at each concentration.
Standards Reference
Assays are designed with reference to ISO 10993-5, adapting exposure conditions and cell models to suit dietary supplement contexts. This ensures familiar, auditable practice for QA and partner reviews.
Test Design Highlights
- Dose range: at least five concentrations spanning the anticipated use range; more can be added for finer resolution.
- Replicates: technical replicates per dose to ensure statistical robustness.
- Controls:
- Negative control (vehicle/medium) to define 100% viability.
- Positive control (known cytotoxic agent) to confirm assay sensitivity.
- Interference checks: visual and instrument checks for color or turbidity that may affect optical readouts; mitigation strategies (e.g., blanks, dilution, alternative reading wavelength) discussed at scoping.
Outputs You Receive
- % cell viability at each concentration (tabulated).
- Dose-response curve with fitted model and confidence shading.
- IC50 value, where the response permits reliable estimation.
- Assay acceptance summary (control performance, variability).
- Interpretive statement explaining what the data imply for safe formulation ranges and whether further testing is advisable.
How We Work With You
1) Scoping & Study Plan
A short technical discussion aligns on product type, intended use, target dosage, and matrix considerations (e.g., sugars, oils, acidity). We agree on:
- Cell model and exposure time appropriate to the scenario.
- Concentration series (≥ five points) and replication plan.
- Sample preparation: dissolution, dispersion, or extraction in a suitable medium.
- Any special notes (e.g., light sensitivity) to ensure valid results.
2) Sample Receipt & Preparation
Upon arrival, samples are logged and stored per your instructions. If a tablet or capsule needs disintegration before testing, we prepare test solutions and document each step so results are reproducible and auditable.
3) Assay Execution
Cells are seeded and equilibrated; test solutions are added across the concentration range. After the planned exposure, we conduct the MTT readout, solubilize formazan, and read absorbance.
4) Data Analysis
We normalize absorbance values to controls, plot dose–response, and calculate IC50 (if applicable). Acceptance criteria for controls and curve fits are applied before finalizing results.
5) Reporting & Review
You receive a decision-ready report (PDF) and, on request, raw data spreadsheets. We also include practical guidance for example, suggested concentration windows for further formulation work.
Practical Use Cases
- Early screening of new botanical suppliers. Compare lots and extraction methods to select inputs with acceptable cytotoxicity profiles.
- Setting safe inclusion levels. Use IC50-anchored evidence to define starting percentages for prototypes.
- Change control. Confirm that a process change (e.g., new solvent or carrier) does not increase cytotoxicity.
- Complaint investigation. Combine cytotoxicity results with stability data to identify potential drivers of intolerance.
- Registration support. Include the report in your scientific dossier to show due diligence in safety assessment.
What Makes Supplement Testing Unique and How We Address It
Complex matrices. Powders, syrups, and herbal extracts can be highly colored or turbid. We evaluate interference risk up front and use blank corrections or agreed dilution ranges so results remain valid.
Dose realism. Where possible, we test concentration ranges that reflect intended human exposure after product reconstitution or digestion-like dilution, ensuring results are meaningful for the label dose.
Reproducibility. We document preparation steps (e.g., disintegration time, solvent ratios), so you and your partners can replicate the study or use our settings as a benchmark for supplier qualification.
What’s in the Report
- Executive Summary – Objective, method reference (ISO 10993-5), and headline conclusion.
- Materials & Methods – Cell line, exposure time, media, vehicle, dose series, controls, acceptance criteria.
- Results – Tables of % viability, dose–response graphs, IC50 where calculable.
- Observations – Notes on color/turbidity, precipitation, pH, or odor; any mitigations applied.
- Interpretation & Guidance – Practical statements about safe ranges to explore and whether further studies (e.g., absorption or bioactivity modules) would be appropriate.
- Appendix – Raw absorbance values, curve-fit diagnostics, and versioned SOP references.
The layout is concise enough for partner sharing but detailed enough for QA review and regulatory files.
Tips for Submitting Samples
- Quantity: enough material to complete the concentration series with replicates and repeats (we confirm in the quotation stage).
- Packaging: clean, inert containers; avoid cross-contamination (especially with strong fragrances or essential oils).
- Labeling: sample name, lot/batch, form (powder, liquid), and concentration if a solution.
- Documentation: any known solubility, pH, or stability constraints; recommended vehicles (e.g., water, ethanol-water, buffer); storage instructions.
- Intended dosage: your planned serving size or inclusion percentage helps us place the dose range sensibly.
FAQs
Is this test accepted for product registration?
The assay is designed with reference to ISO 10993-5 and is widely recognized for in vitro cytotoxicity assessment. It provides objective safety evidence that can be included in scientific dossiers. Always confirm the exact requirements of your target market.
Can highly colored extracts be tested?
Yes. We discuss potential optical interference during scoping and apply mitigations (blanking, dilution, or alternative read settings) to maintain data integrity.
Do you test probiotics or live organisms?
Cytotoxicity focuses on effects of substances on mammalian cell viability. For live microbes, please contact us to discuss scope and fit before sending samples.
Can you test the whole finished product?
Yes. We can prepare test solutions from capsule or tablet contents, reconstitute powders, or dilute liquid shots according to your instructions, then run the assay.
What if I also need absorption or bioactivity information?
Our broader supplement testing support includes absorption/bioavailability and bioactivity evaluations. Once safety screening is complete, we can recommend suitable next steps based on your goals.
How to Start
- Share your goal: product type, intended dose, and whether you’re screening ingredients or final formulas.
- Receive a study plan & quotation: includes dose range, replication, and any special handling.
- Send samples: follow the submission tips to avoid delays.
- Get results: a decision-ready report with % viability and IC50, plus interpretation aligned with your objective.
Cytotoxicity testing is the fastest, most ethical way to de-risk dietary supplements before launch. NANOTEC’s service uses non-animal, ISO-aligned methods and the MTT assay to quantify cell viability and estimate IC50 across a defined dose range. With expert consultation, careful method design, and clear reporting, you gain the scientific confidence to finalize formulations, support registration, and communicate safety to customers and partners.


