Meta description (140–160): Evaluate chromosomal damage with NANOTEC’s in vitro micronucleus test (OECD 487). Non-animal, standards-aligned genotoxicity data for cosmetics, health products, and dietary supplements, decision-ready reports with expert guidance.
SEO Keywords: in vitro micronucleus test, OECD 487, genotoxicity testing Thailand, cosmetics safety testing, dietary supplement genotoxicity, CBMN assay, chromosomal damage screening, non-animal safety assessment, clastogenicity aneugenicity, S9 metabolic activation, NANOTEC testing services
Service Overview

When a cosmetic, health product, or dietary supplement introduces a new active, higher use level, or novel extraction/processing route, developers must verify that it does not induce chromosomal damage. The in vitro micronucleus test (MN test) provides exactly this evidence. By scoring the formation of micronuclei, small, additional nuclei created from chromosome fragments or whole chromosomes that fail to segregate during cell division, the assay detects clastogenic (chromosome-breaking) and aneugenic (chromosome loss/gain) effects.
At the National Nanotechnology Center (NANOTEC), our MN test is designed with reference to OECD Test Guideline 487, giving you non-animal, internationally recognized genotoxicity data that support R&D decisions, specification setting, risk assessment, and regulatory submissions. The method is relevant for botanical extracts, finished products, concentrates, and purified actives across both cosmetics/health products and dietary supplements.
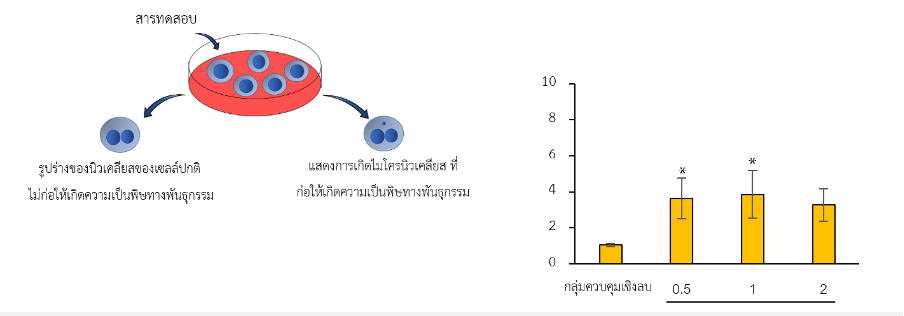
What the Micronucleus Test Measures
During mitosis, chromosomes should segregate cleanly into daughter nuclei. Certain substances can damage chromosomes (creating fragments) or disrupt the mitotic apparatus (causing entire chromosomes to lag). These pieces or lagging chromosomes become enclosed by nuclear membrane material, forming micronuclei, smaller “satellite” nuclei visible beside the main nucleus. A higher frequency of micronuclei in treated cells, compared with negative controls, flags genotoxic potential under the test conditions.
Who This Service Is For
- Cosmetics & personal care brands introducing new actives, higher dosages, or novel delivery systems (e.g., encapsulated botanicals, nano-enabled formats).
- Health product developers (topicals, oral products) who require non-animal chromosomal damage screening aligned to recognized standards.
- Dietary supplement companies and ingredient suppliers needing OECD-aligned evidence for botanical extracts, standardized fractions, or bioactive complexes.
- Contract manufacturers (ODM/OEM) who must qualify suppliers, verify batch changes, or provide scientific dossiers to clients.
- R&D teams seeking an early, reliable go/no-go genotoxicity signal before expanding investment.
Why Choose NANOTEC
Standards-aligned method, non-animal approach.
We design and execute the MN test with reference to OECD 487, using validated mammalian cell systems and clearly documented procedures, without animal use.
Cross-functional guidance.
From sample preparation (solubility, pH, vehicles) through dose-range selection and interference control (color/turbidity), our researchers collaborate with you so the data reflect realistic product use and meet your documentation needs.
Decision-ready outputs.
Results are delivered with micronucleus frequency, concurrent cytotoxicity indicators, acceptance checks, and interpretation, organized for regulatory files, QA review, and partner communication.
Complementary safety testing under one roof.
If you also need in vitro cytotoxicity (e.g., MTT, % viability, IC50) or follow-on assays in your safety program, we can scope those sequentially to build a coherent evidence package.
Scope & Pricing
- In vitro micronucleus test (OECD 487 reference): starting at THB 90,000 per sample
Final cost depends on study design choices (e.g., with/without metabolic activation, number of exposure conditions, concentration tiers, replication).
Sample types supported
- Raw actives (purified compounds, standardized herbal actives).
- Botanical extracts (solvent extracts, CO₂ extracts, enriched fractions).
- Finished products (creams/serums, tonics, capsules/tablets contents, powders, syrups, shots).
- Concentrates or premixes used at low in-use dosage.
A detailed quotation follows a short technical scoping discussion to confirm design elements and any matrix-specific handling.
Method Summary (Aligned to OECD 487)
Core principle
Cells are cultured and exposed to the test article across a dose-response series. After exposure, the assay preferentially scores binucleated cells (via cytokinesis-block protocols) to ensure each evaluated cell has completed one mitotic division, making micronuclei attributable to the test period. Micronucleus frequency in treated groups is compared to concurrent controls.
What the assay detects
- Clastogenicity: chromosome breaks, producing acentric fragments.
- Aneugenicity: disruption of the spindle apparatus or chromosomal segregation, causing whole chromosomes to lag.
Metabolic activation (when relevant)
Where warranted by the chemistry or intended exposure route, the study can include conditions with metabolic activation (e.g., S9 mix) and without metabolic activation to capture pro-genotoxicants requiring bioactivation.
Controls & acceptance
- Negative/vehicle controls define baseline micronucleus frequency.
- Positive controls recommended by the guideline demonstrate method sensitivity to clastogens and/or aneugens.
- Pre-specified acceptance criteria (e.g., control performance, cytotoxicity boundaries, scorable cell counts) ensure data validity.
Study Design: What We Align With You
1) Cell model & exposure scenario
Selection of a validated mammalian cell system (e.g., human lymphocytes or an appropriate cell line) and exposure duration consistent with OECD 487 and your product context (e.g., short treatment ±S9; longer treatment without S9).
2) Dose-range and spacing
At least three analyzable concentrations are targeted, typically bracketed by a non-toxic low dose and a limit of acceptable cytotoxicity at the high end (while considering solubility/precipitation and pH). A pre-range-finding step can be used for unfamiliar matrices.
3) Replication & scorable events
Replicates are incorporated to support statistical confidence and ensure a sufficient number of binucleated cells are scored per condition, in line with guideline expectations.
4) Cytotoxicity indicators
Concomitant measures (e.g., cytokinesis-block proliferation index) support interpretation, ensuring micronucleus increases are not confounded by excessive cytotoxicity.
5) Interference management
Highly colored, opaque, or particulate samples (common with herbal concentrates) are handled with predefined strategies (e.g., filtration where appropriate, vehicle optimization, or dose limitation to avoid artifactual scoring issues).
What You Receive: Reporting & Deliverables
- Executive summary: objective, OECD 487 reference, design overview, headline conclusions.
- Methods: cell model, exposure conditions (±S9 as applicable), dose series, replication, controls, acceptance criteria.
- Results:
- Micronucleus frequency for each concentration and control (tabulated).
- Cytotoxicity indicators and observations (e.g., precipitation, pH, coloration) relevant to data quality.
- Graphs illustrating dose–response trends.
- Interpretation: clear statements on clastogenic/aneugenic signal under the conditions tested, including practical guidance (e.g., “proceed,” “reformulate,” or “retest at adjusted range”).
- Appendices: raw scores or summary spreadsheets; any microscope/photo documentation if arranged.
The format is concise for partner sharing yet sufficiently detailed for QA and regulatory reviewers.
Where the MN Test Fits in Your Safety Program
- Early-stage screening: De-risk novel actives or supplier changes before scale-up.
- Specification setting: Use the absence of a micronucleus signal (within cytotoxicity boundaries) to support acceptable concentration ranges.
- Change control: Confirm that process changes (e.g., extraction solvent, stabilizers, encapsulation) do not introduce genotoxic concern.
- Dossier building: Combine micronucleus results with cytotoxicity and other non-animal endpoints to build a coherent, science-based safety narrative.
Samples: Preparation & Submission Tips
Quantity & format
Send enough material for the planned dose series, replicates, and potential repeats. We will confirm quantities at quotation. Provide powders, concentrates, or finished product in clean, inert containers.
Documentation
Include: product name/ID, lot/batch, known solubility and pH preferences, recommended vehicles (e.g., water, buffer, ethanol-water), storage instructions, and any light/temperature sensitivity notes.
Matrix-aware guidance
If your product contains strong colorants, essential oils, or particulates, mention this at scoping so we can pre-plan the dose range and handling to protect data integrity.
Practical Examples
- Cosmetic serum with a novel botanical complex: Micronucleus test (±S9 as appropriate) at concentrations reflecting in-use exposure in skin-relevant media.
- Encapsulated herbal active for oral supplements: Test the active and/or the finished powder/liquid across a justified range, with attention to solvent choice and pH to avoid artifacts.
- Process change: A switch to a new extraction solvent, verify no new genotoxic signal appears in the finished concentrate.
Frequently Asked Questions
What exactly does the micronucleus assay detect?
It detects chromosomal damage resulting in micronuclei, small, extra nuclei derived from chromosome fragments (clastogenicity) or whole chromosomes that fail to segregate (aneugenicity).
Is the test animal-free?
Yes. The assay uses cultured mammalian cells and is designed with reference to OECD 487, a recognized in vitro guideline.
Do you include metabolic activation?
Where relevant to the chemistry or use scenario, we can run with and without S9 metabolic activation to capture parent and metabolite risks. This is finalized during scoping.
How many doses are tested?
We target at least three analyzable concentrations within acceptable cytotoxicity limits, with additional levels as needed to resolve a trend. Exact design is confirmed per sample.
My extract is highly colored, can that interfere?
Color/turbidity can complicate some readouts; we plan interference mitigation (e.g., vehicle optimization, filtration where appropriate, dose limits) so micronucleus scoring remains reliable.
Will the result guarantee regulatory approval?
No single test guarantees approval. However, an OECD-aligned MN test is a strong, widely recognized piece of evidence within a modern, non-animal safety assessment.
How We Work With You
1) Scoping & quotation
We review your product, intended use level, and matrix properties, then propose an OECD-aligned design (±S9 as relevant), dose series, and replication, together with a firm quotation.
2) Sample receipt & preparation
We log, store, and prepare samples per your instructions (e.g., dissolution in appropriate vehicle, pH adjustment if justified). Any special handling is documented.
3) Assay execution & quality checks
Cells are exposed under the agreed conditions. Negative and positive controls confirm method performance; cytotoxicity indicators ensure results are interpreted within acceptable biological limits.
4) Analysis & reporting
We compile a decision-ready report with micronucleus frequencies, cytotoxicity observations, acceptance checks, and a clear interpretation in the context of your objective.
Pricing & Options
- In vitro micronucleus test (OECD 487 reference): from THB 90,000 per sample
- Options may include with/without S9, expanded dose levels, additional replication, or supplemental documentation (e.g., image sets).
- A tailored quotation follows scoping.
Suggested Internal Links
- Cytotoxicity Testing for Cosmetics & Health Products (ISO 10993-5, MTT)
- Cytotoxicity Testing for Dietary Supplements (ISO 10993-5, MTT)
The in vitro micronucleus test is the gold-standard non-animal screen for chromosomal damage, detecting both clastogenic and aneugenic effects by measuring micronuclei in dividing cells. At NANOTEC, we run this assay with reference to OECD 487, tailoring study design to the realities of cosmetic, health product, and dietary supplement matrices. You receive clear, defensible results micronucleus frequencies, cytotoxicity context, controls, and interpretation,organized for R&D decisions, QA, and regulatory files.
If you are introducing a new active, pushing concentrations, changing extraction processes, or qualifying suppliers, the MN test is a pivotal step in a science-based, non-animal safety program.


